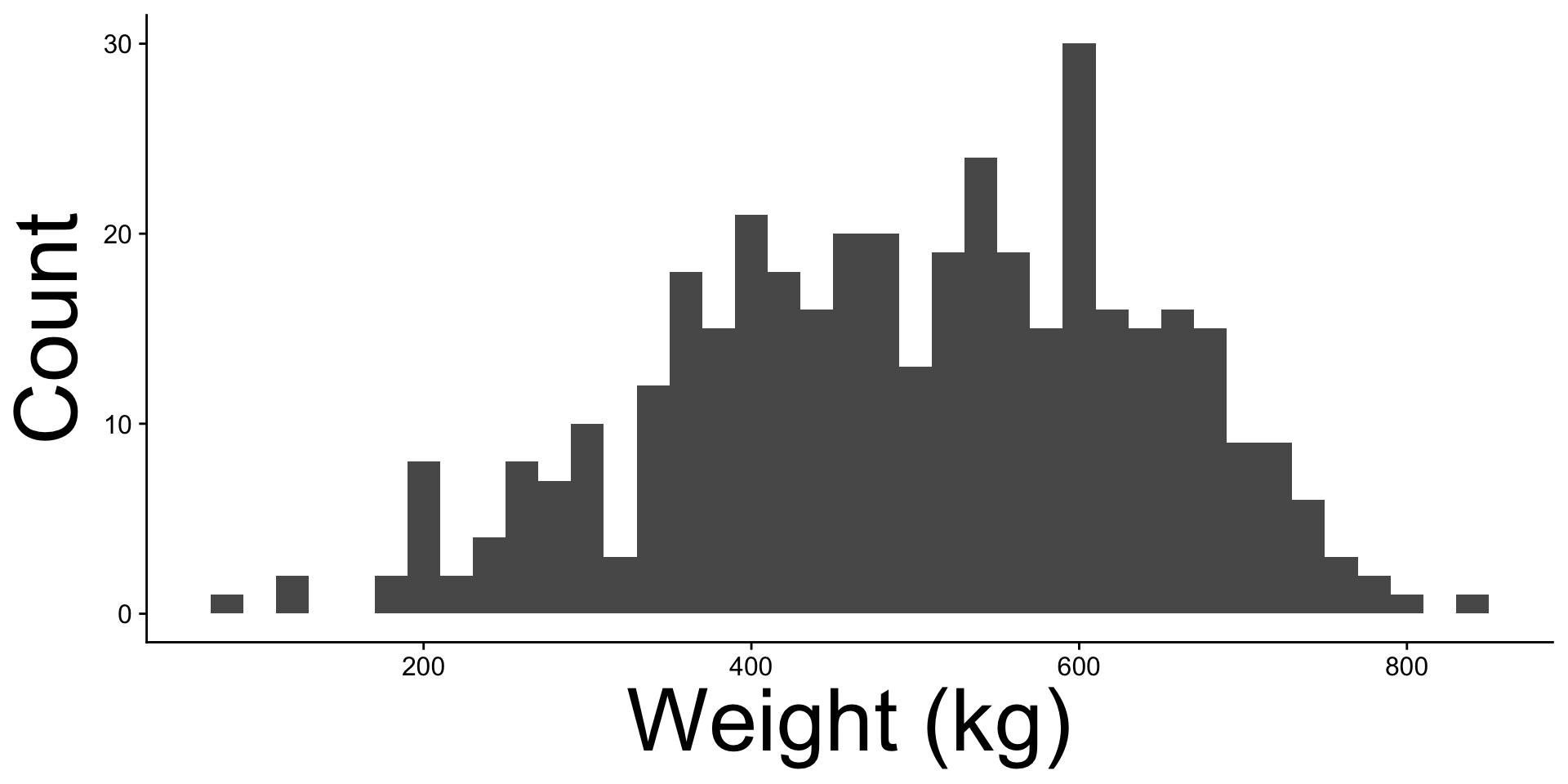
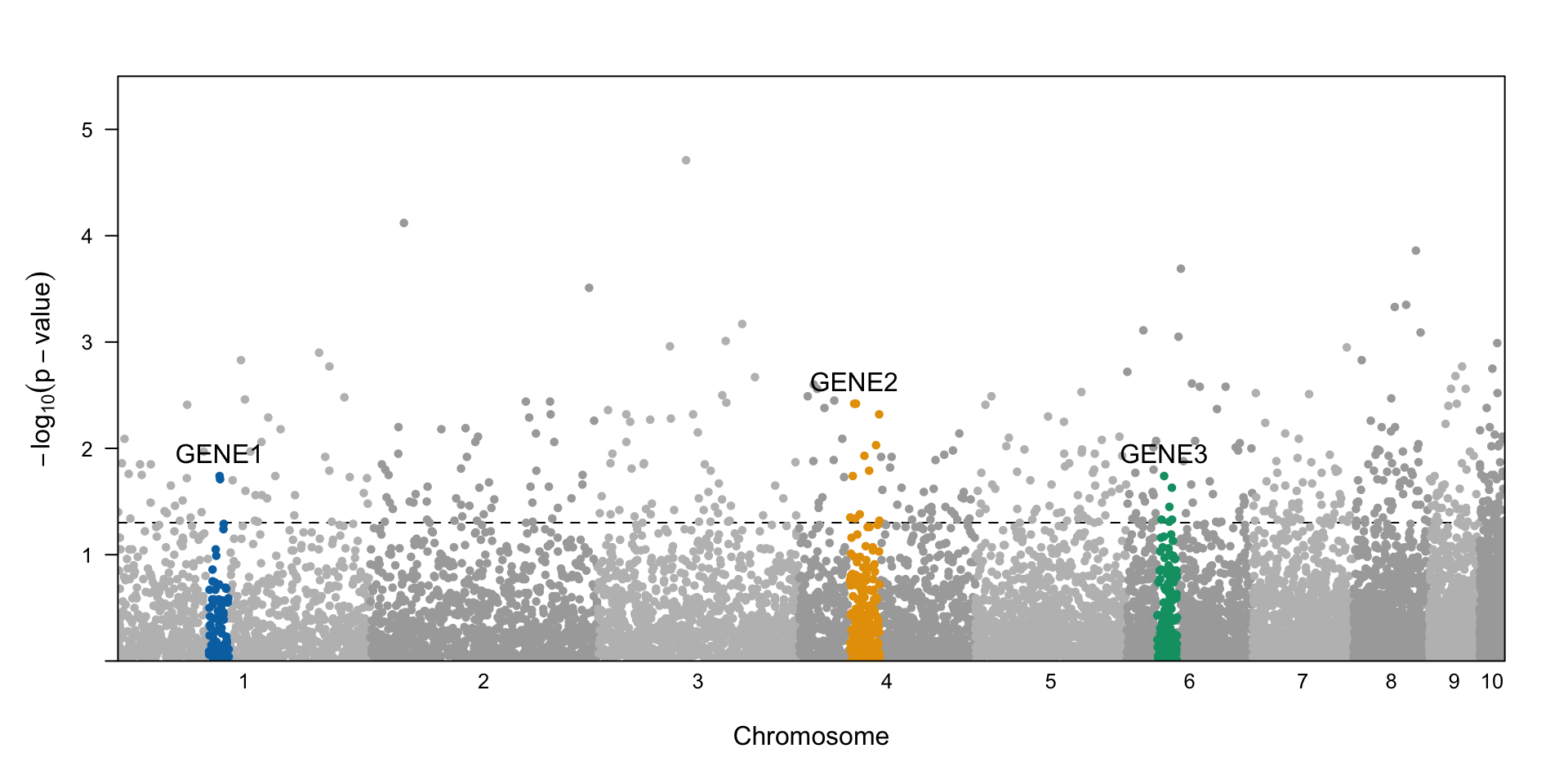
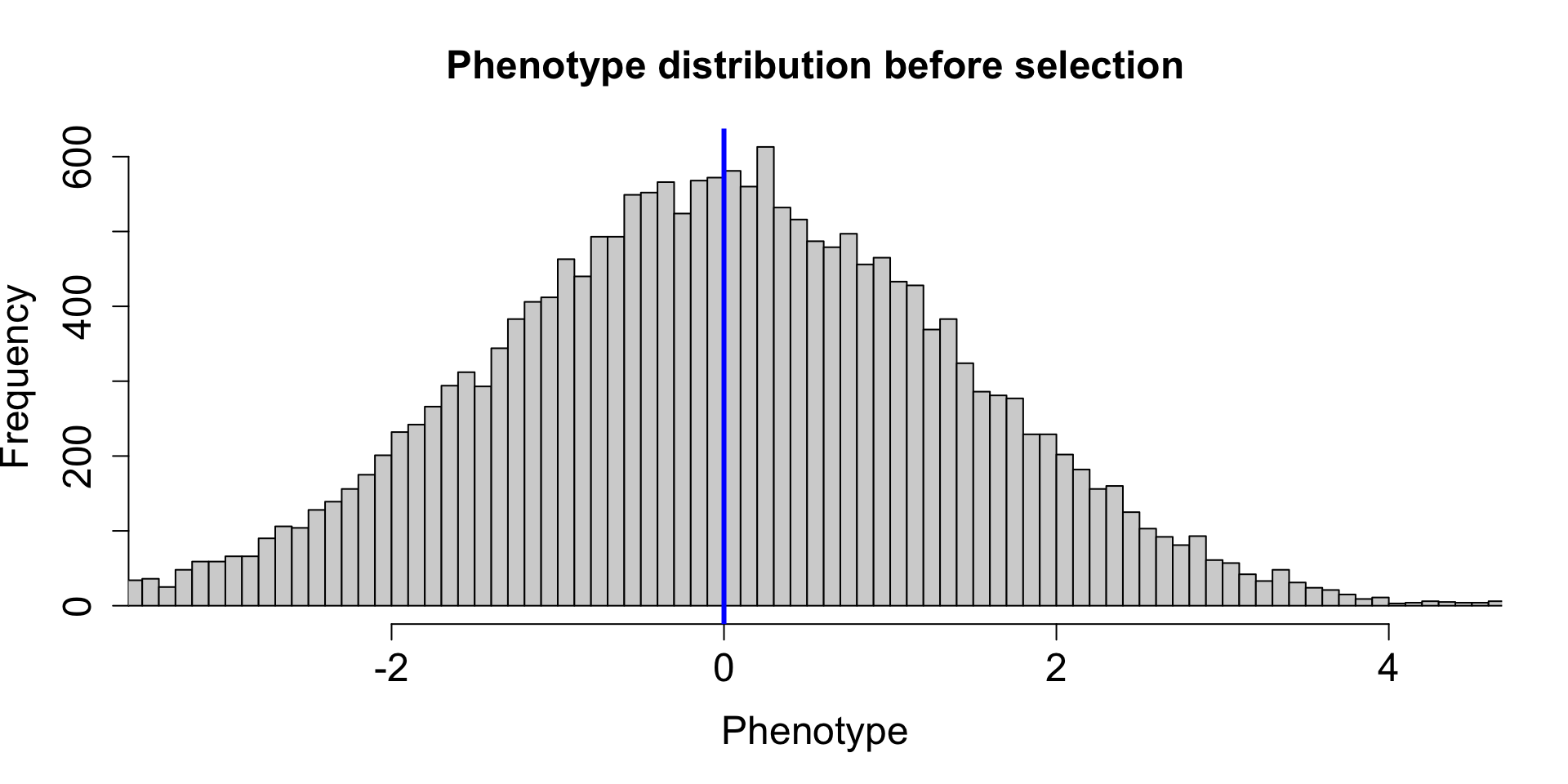
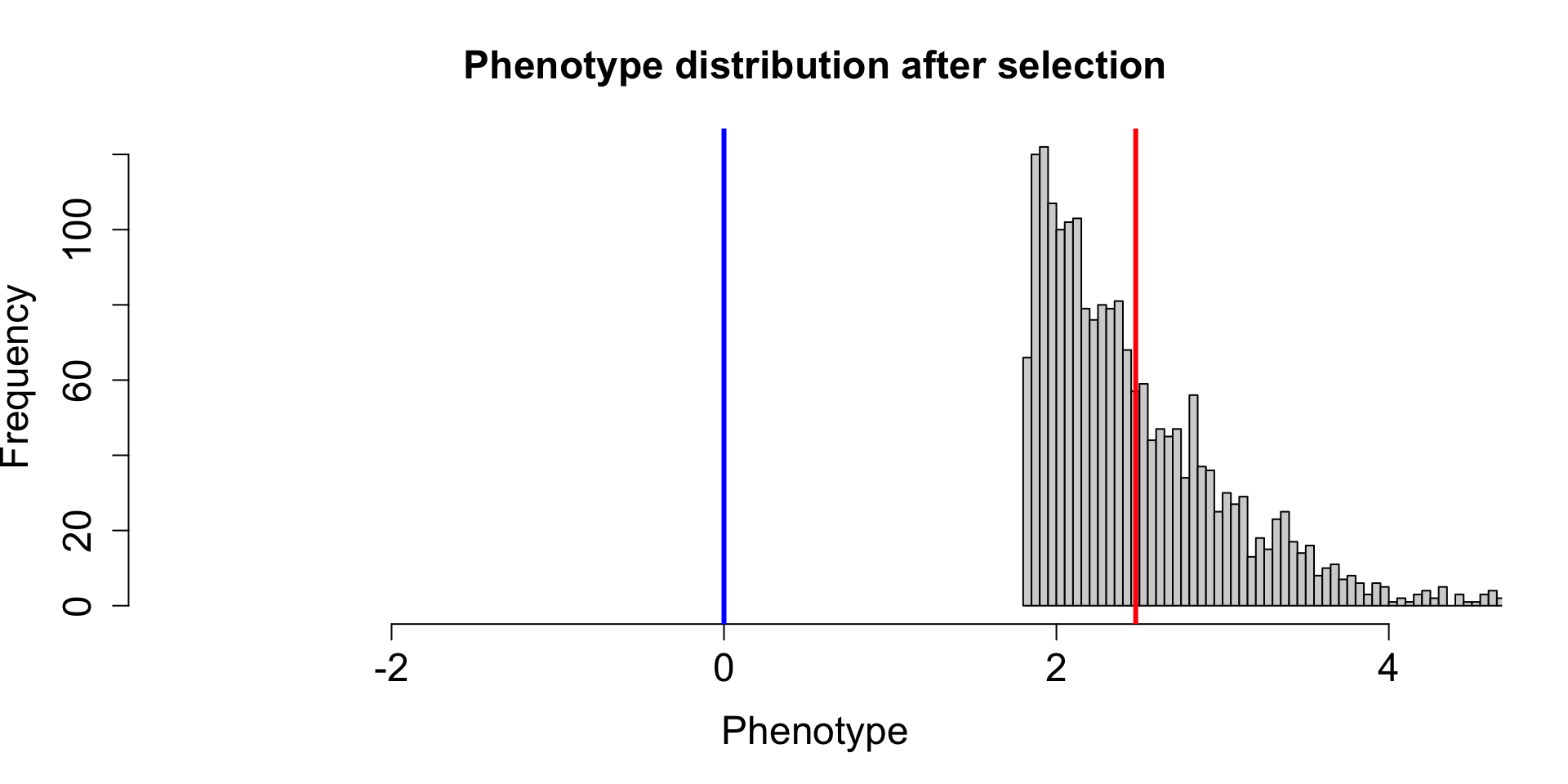
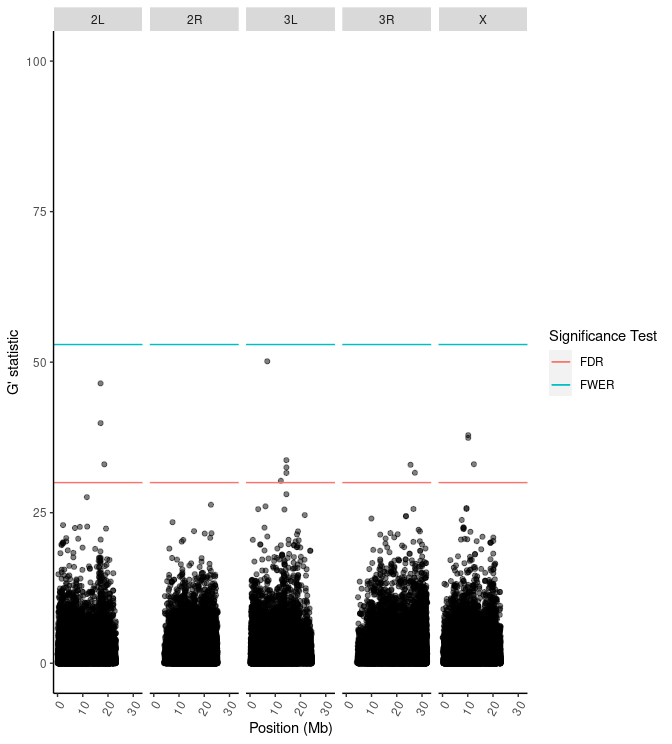
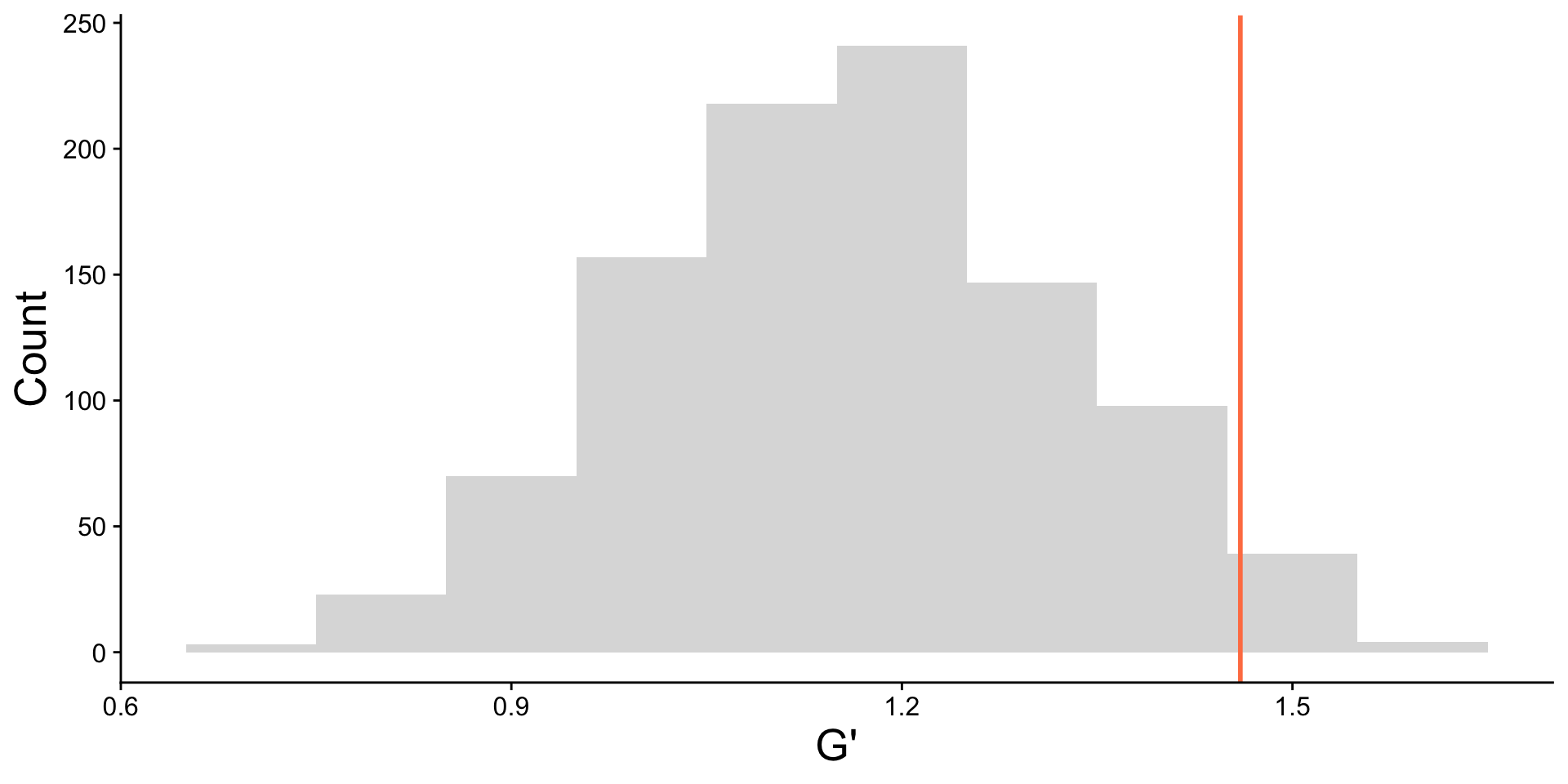
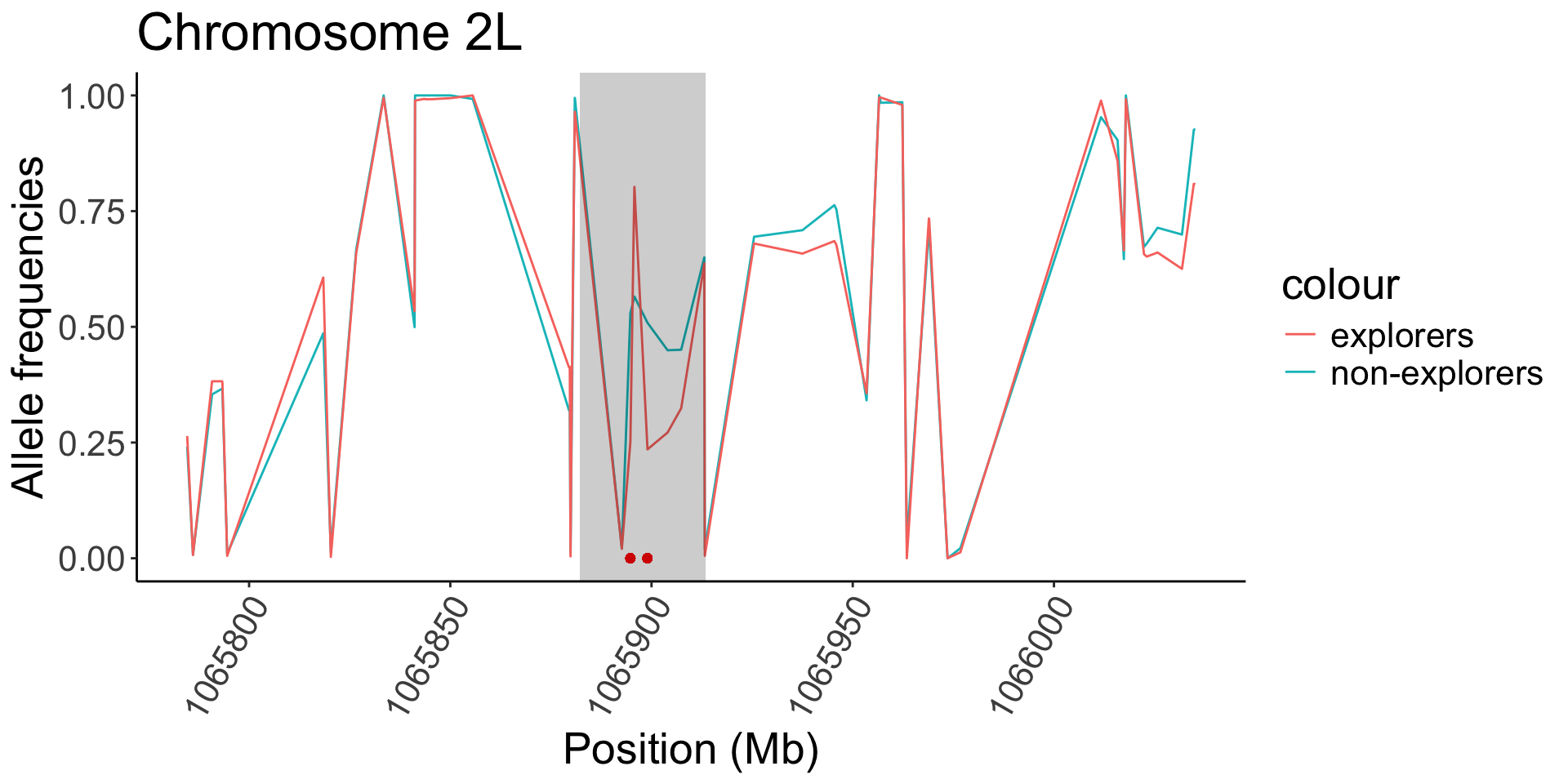
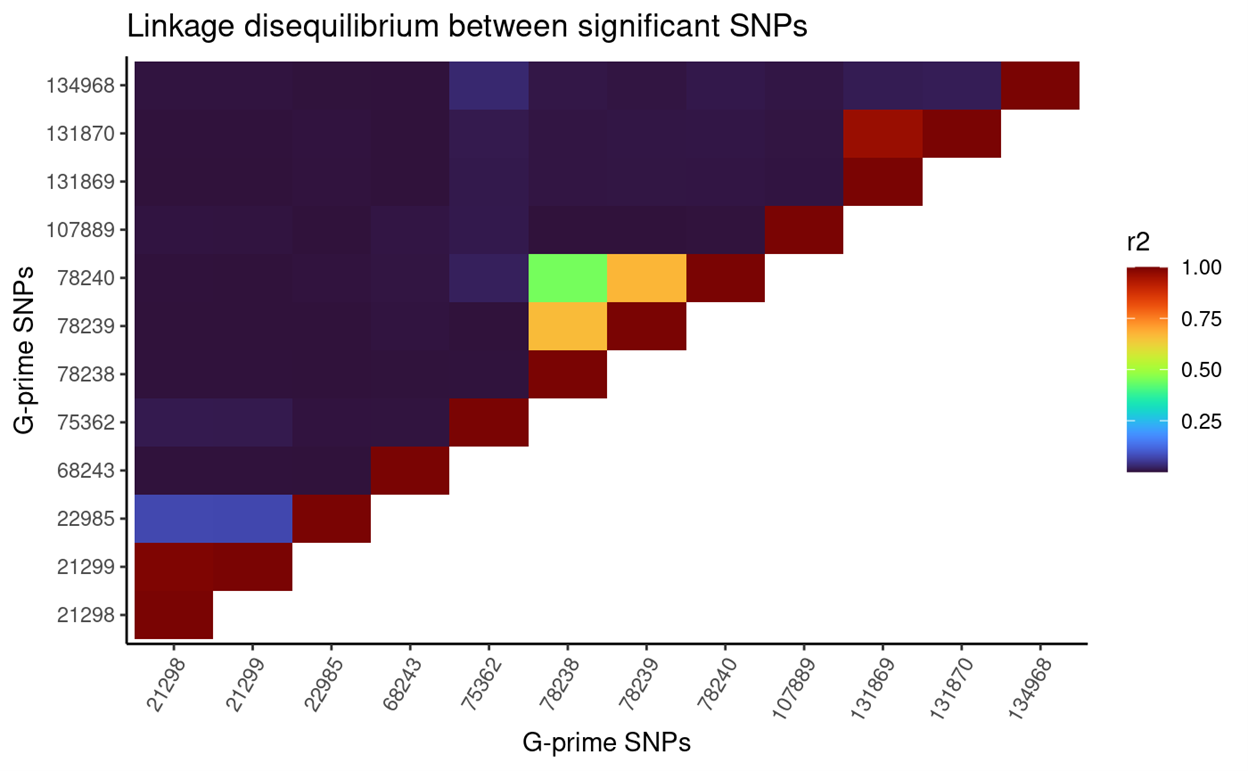
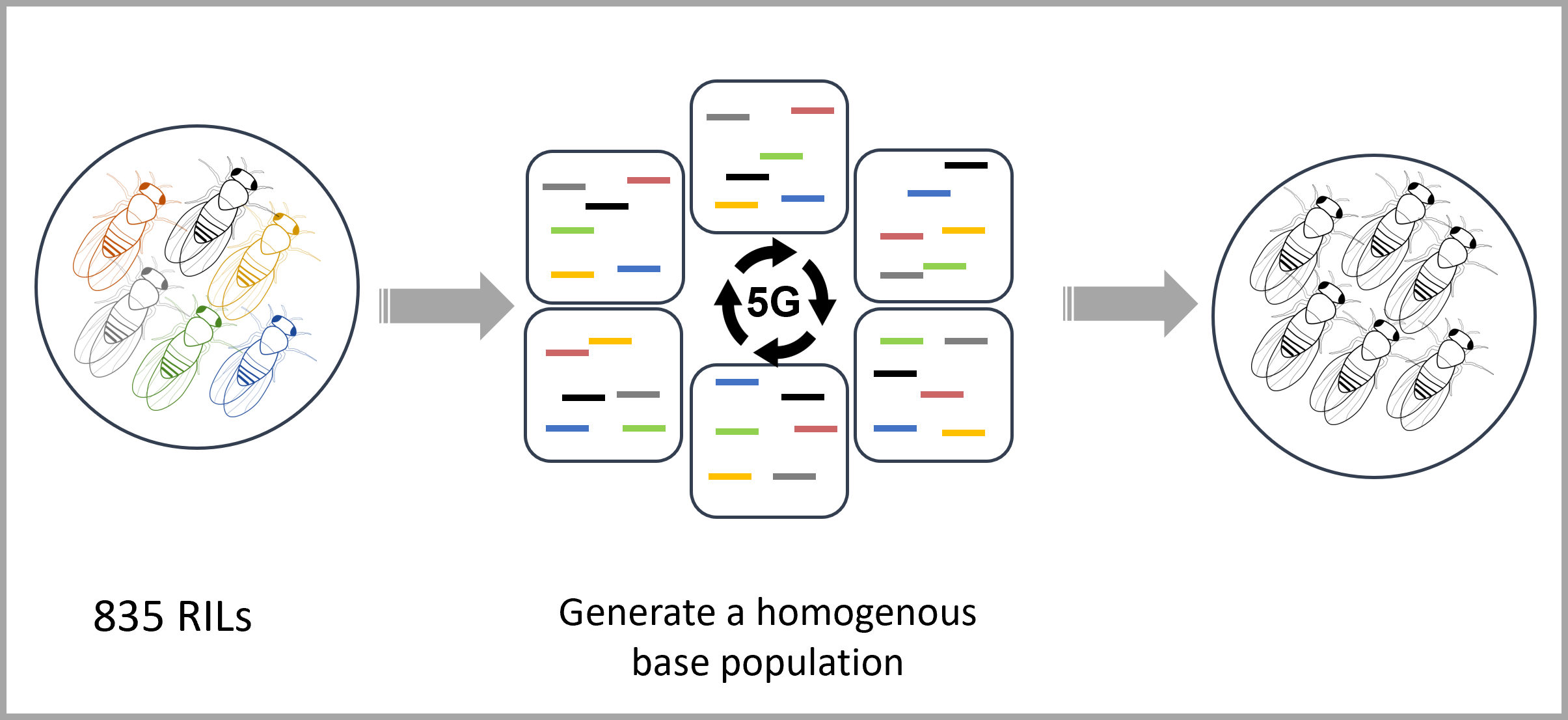
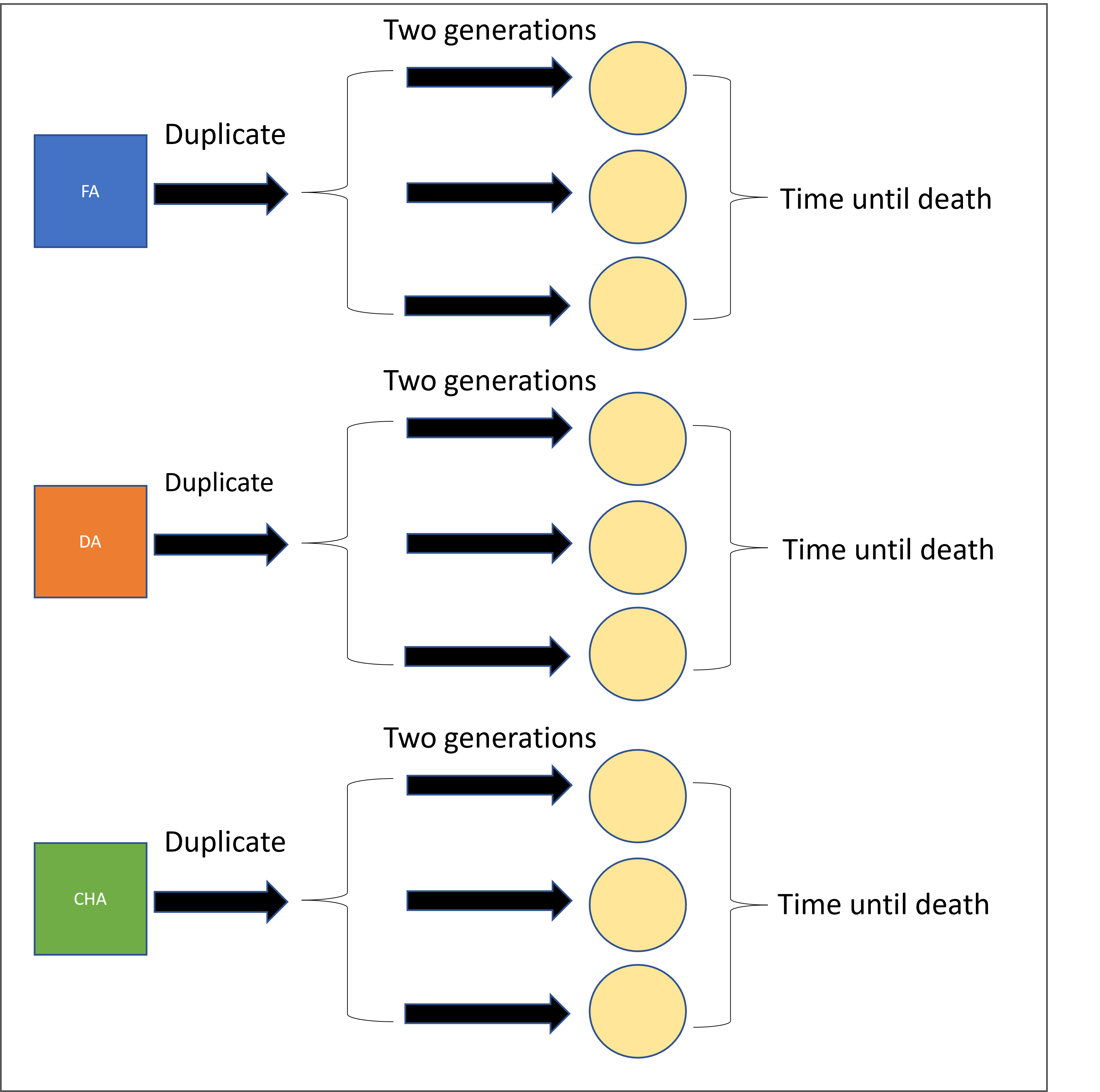
| 2L |
17054316, 17054384 |
CG15136 |
Abnormal flight |
|
18515161 |
Ugt201D1 |
Enables UDP-glycosyltransferase activity |
|
|
CG10211 |
Involved in response to oxidative stress |
| 3L |
6818526 |
vvl |
Specification of cell fates, patterning and immune defense |
|
12226612 |
app |
Regulation of fat signaling, abnormal locomotive behavior |
|
14401683, 14401693, 14401703 |
Dscam2 |
Abnormal neuroanatomy, size, body color |
| 3R |
25454633 |
Men |
Abnormal heat stress response, abnormal sleep |
|
27194130 |
G14369, CG14370 |
Little to no information |
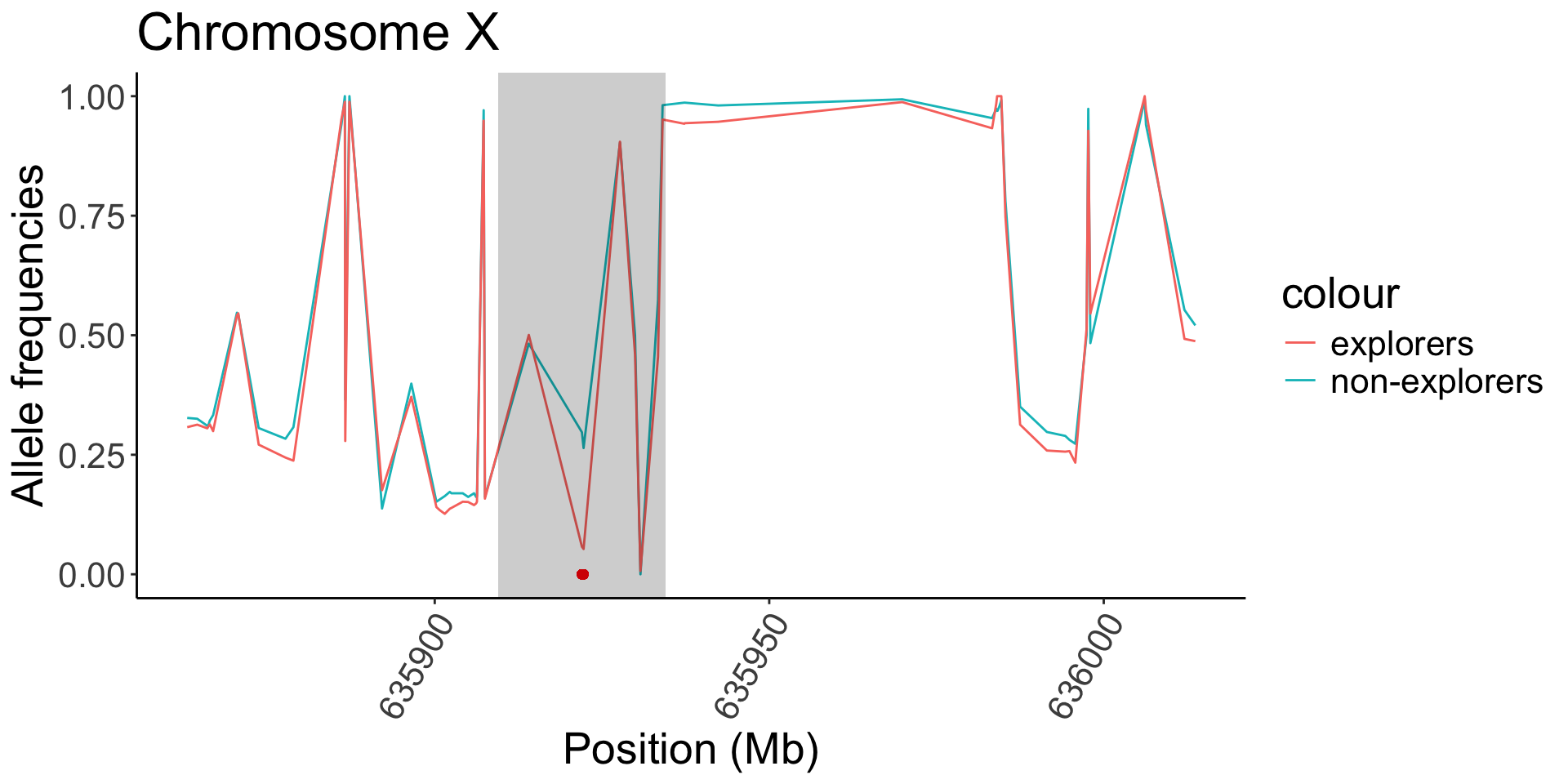
| X |
10174752, 10174756 |
CG32767 |
Expressed in wing hinge primordium and wing pouch |
|
12446014 |
Btnd |
Flightless, abnormal heat stress response |
|
|
Efr |
Manifests in wing vein |
|
|
sqh |
Involved in cytokinesis and tissue morphogenesis |
|
|
dtn |
Abnormal heat stress response |